Dissolution 1-on-1
Dissolution 1-on-1 (sponsored by Agilent Technologies) is a highly interactive package which provides theoretical knowledge of how and why dissolution testing is performed. The package is available in English, Spanish and Chinese. The package consists of the activities:
- Foreword
- Chapter 1: Introduction To Dissolution Testing
- Chapter 2: The Dissolution Apparatus – Anatomy
- Chapter 3: Critical Physical Parameters
- Chapter 4: Performing The Dissolution Test
- Chapter 5: Dissolution Apparatus Qualification
- Chapter 6: Dissolution And Automation
- Chapter 7: Reference Information
This package also contains two different Assessment modules with MCQs. See ‘Contents’ tab for more information.
Product Code: COP007
Price: £300.00 p.a. (per institution)
Dissolution 1-on-1 consists of the following main activities/chapters:
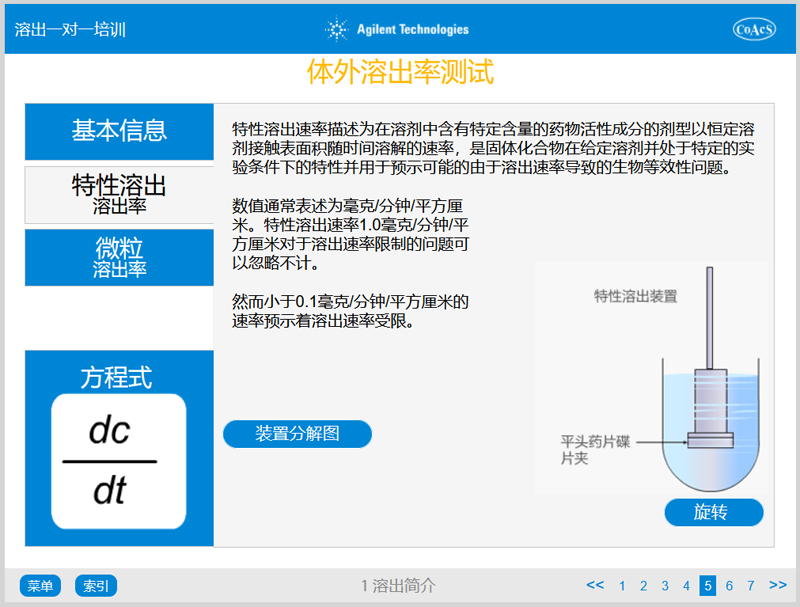
Chapter 1: Introduction To Dissolution Testing
Chapter 1 covers information which provides a basic understanding of:
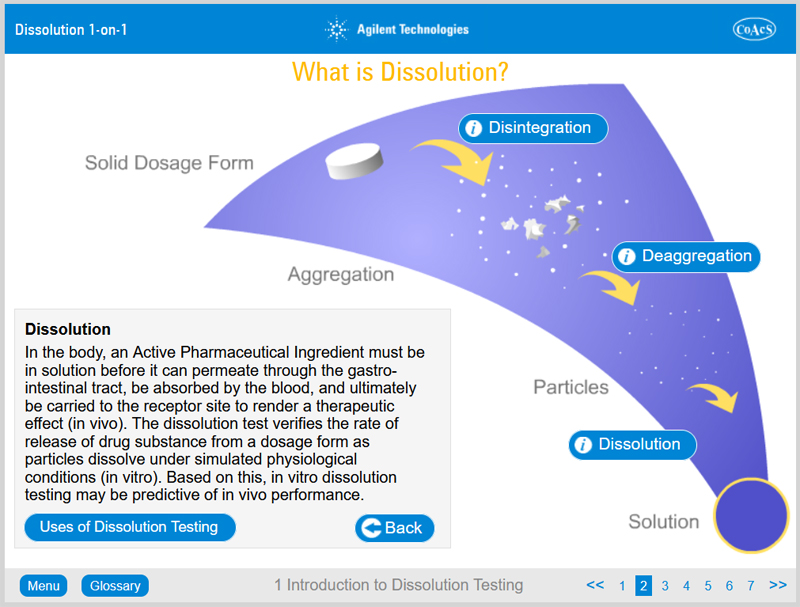
- The process of dissolution
- In vitro in vivo correlation
- The concept of dissolution rate and associated equations
- The history of dissolution testing
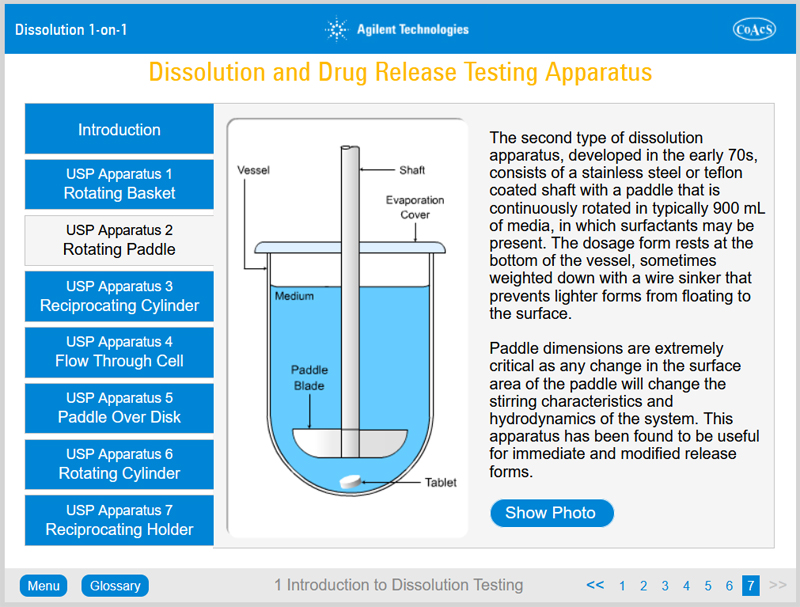
- The different types of apparatus used in dissolution testing
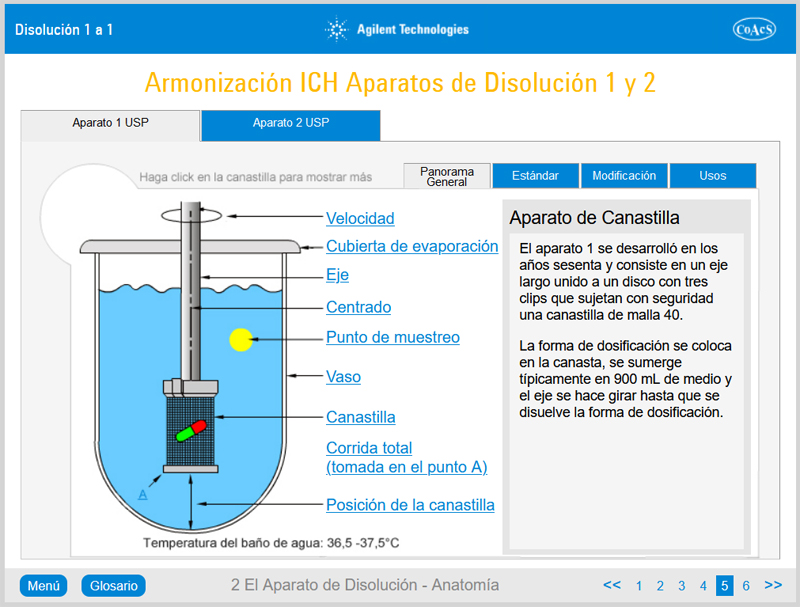
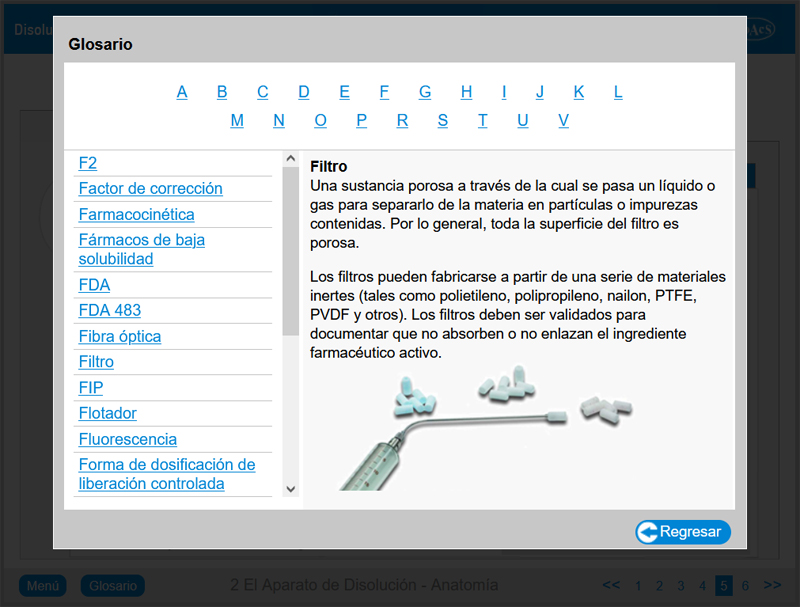
Chapter 2: The Dissolution Apparatus – Anatomy
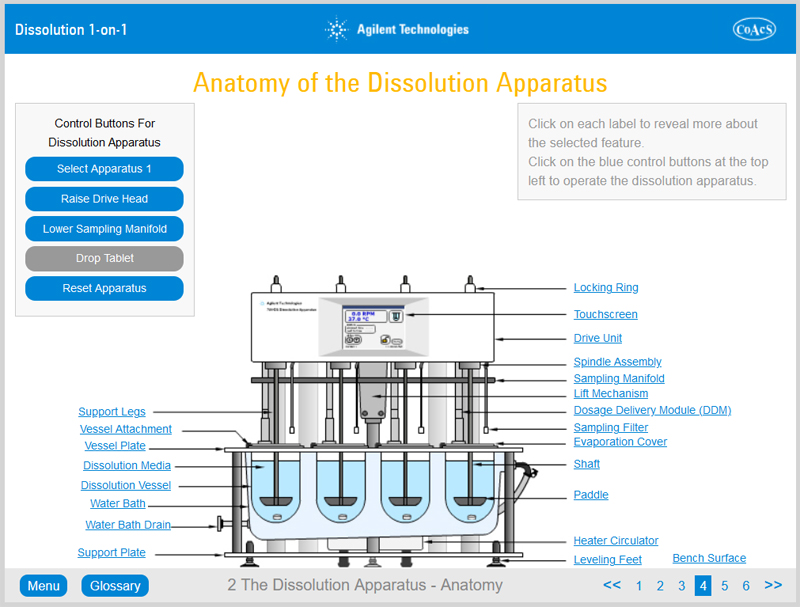
Chapter 2 provides the user with in-depth knowledge of the features and operation of Apparatus 1 (rotating basket method) and Apparatus 2 (rotating paddle method). Upon completion of this chapter, the user will be familiar with all of the features of a typical dissolution apparatus.
Chapter 3: Critical Physical Parameters
On completion of Chapter 3, the user will be aware of critical physical parameters of Apparatus 1 and Apparatus 2 that affect dissolution tests, and know how to complete an operation checklist.
Chapter 4: Performing The Dissolution Test
On completion of Chapter 4, the user will:
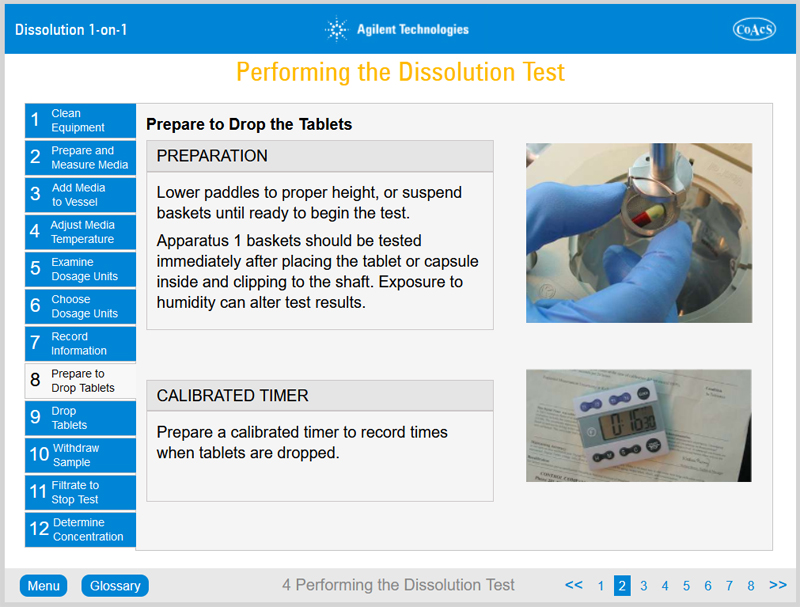
- Be familiar with how to perform a dissolution test using Apparatus 1 and 2
- Be able to calculate sample concentration
- Know the test acceptance criteria governing a variety of dosage forms
- Have an appreciation of non-compendial modifications to both types of apparatus
- Know what to do in the event of dissolution test failure and sources of error for dissolution test failure
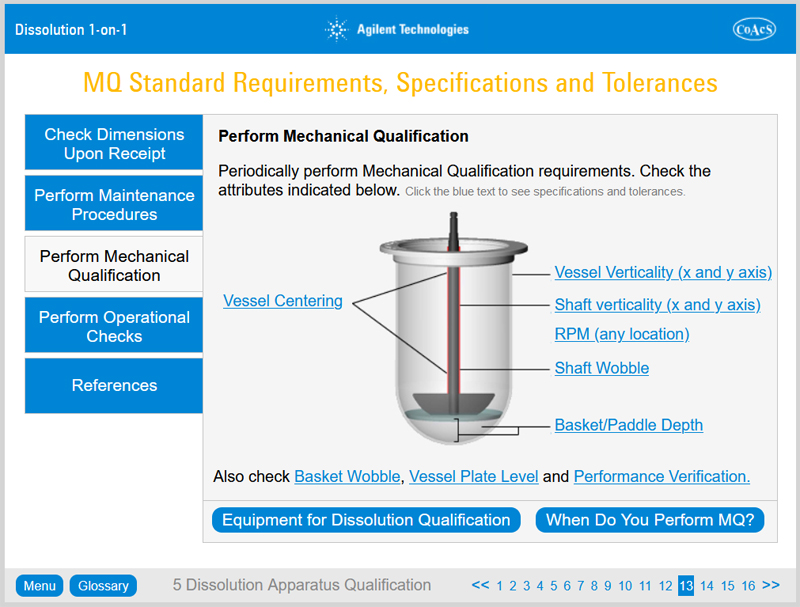
Chapter 5: Dissolution Apparatus Qualification
On completion of Chapter 5, the user will:
- Understand the background to Performance Qualification
- Learn what the Performance Verification Test is, what Mechanical Qualification is and which of them is right for a particular laboratory
- Learn when to perform qualification
- Be aware of the steps involved in filter validation
Chapter 6: Dissolution And Automation
On completion of Chapter 6, the user will be aware of how automation can be used in dissolution testing. The user will be familiar with unit operations, the versatility paradox and be aware of possible future developments in the field of automated dissolution testing.
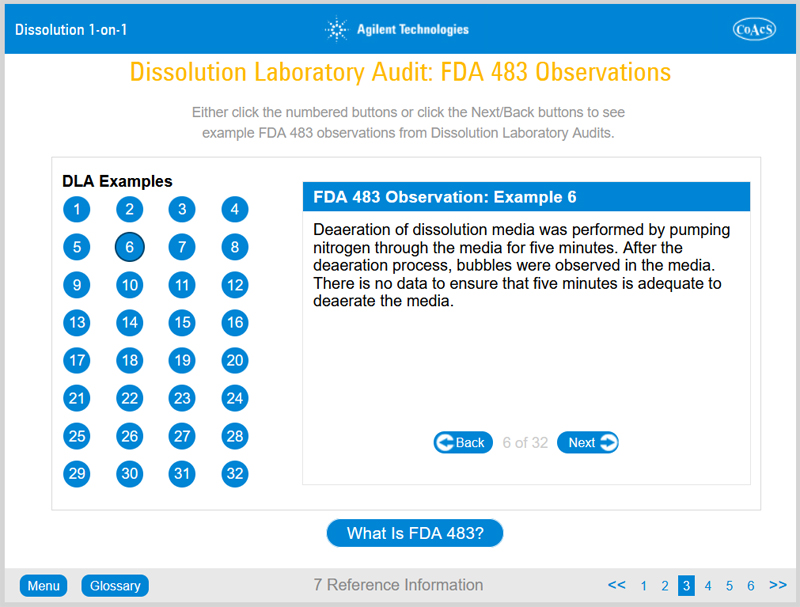
Chapter 7: Reference Information
Chapter 7 provides useful reference information, for example, Dissolution Laboratory Audit: FDA 483 observations, Standard Operating Procedures: FDA 483 observations.
A glossary is available throughout the package.
Information On Assessment And Certification
Two different assessment modules with MCQs are available for Dissolution 1-on-1.
The first assessment module is a simple, informal module with no certification. This informal assessment module is available via the College of Pharmacy website and comes as part of the Dissolution 1-on-1 package licence.
The second assessment module is a formal module with certification. The Dissolution 1-on-1 Training Assessment provides a formal assessment of a user’s knowledge of the material covered in Dissolution 1-on-1, with certificates being issued on successful completion of assessments. Information about the formal assessment and certification is available via a separate website:
Dissolution 1-on-1 is suitable for users working within pharmaceutical organisations and users looking to learn about dissolution. The package provides pharmaceutical organisations with a standardised dissolution training and testing solution.
Pre-requisites: Users need to have no prior knowledge of dissolution although a knowledge of pharmaceutical and biochemical terms would be helpful.
Audience: Users working in pharmaceutical organisations; undergraduate and postgraduate students needing information about dissolution.
Time to complete: 1.5 – 3 hours
There are currently no reviews available for this package.
Share this page
Related resources
Main CoAcS website

The main CoAcS website explains more about the areas in which we operate including:
- pharmacy education
- healthcare applications to hospitals and pharma
- bespoke software
- pharmaceutical plant design and consultancy
College of Pharmacy (CoP)
The College of Pharmacy (CoP) website is the gateway to all of our CAL packages. Besides the subscribed pharmacy learning materials, you will also have free access to the:
- museum – containing images of historical documents and pharmacy artifacts
- library – with listings of the latest pharmacy journals and other publications
- lecture theatre – access to many diverse university presentations
- herb garden – to promote the understanding of herbs and medicinal plants and their place in traditional and modern pharmacy
Pharmaceutical Press agents
We are exclusive agents in the Middle East for the Pharmaceutical Press (the publishing arm of the Royal Pharmaceutical Society). We are able to supply countries with invaluable online resources – such as ‘Medicines Complete’. Many other titles are available including:
- the British National Formulary (BNF)
- Stockley’s Drug Interactions
- Martindale’s Complete Drug Reference
Please contact us directly if you wish to arrange a subscription.