Aseptic Processing
Components arriving for aseptic processing are classed as sterile. During processing of these items to produce the final product, personnel must aim to maintain the sterility of all items used. Contamination therefore needs to be minimised.
The package Aseptic Processing discusses where contamination can arise from, methods and aseptic processing techniques to minimise contamination and methods of environmental monitoring to check for contamination. These methods include:
- Clean Rooms (e.g. design, grading and use of)
- Clean air devices including Horizontal and Vertical Laminar Flow Cabinets
- Techniques for minimising contamination from personnel (e.g. correct hand washing technique, use of appropriate protective clothing)
Multiple choice questions are interspersed throughout the package to test the user’s understanding of the material covered.
Product Code: COP005
Price: £300.00 p.a. (per institution)
OVERVIEW OF THE PACKAGE
Aseptic Processing starts by describing what the main sources of contamination are and continues by discussing the main methods available to control contamination. Methods discussed include:
- Clean Rooms (e.g. design, grading and use of)
- Clean air devices including Horizontal and Vertical Laminar Flow Cabinets
- Techniques for minimising contamination from personnel (e.g. correct hand washing technique, use of appropriate protective clothing)
The package covers a range of methods used to monitor the following environmental aspects of Clean Rooms: the air (e.g. settle plates), the surfaces (e.g. agar sausages) and the personnel (e.g. five finger glove prints).
The last two activities of the package describe aseptic processing techniques. The first of these activities covers techniques that are used in several curricula including microbiology and pharmaceutical processing; this includes general practices that should be adopted and specific techniques such as the preparation of a bacterial lawn. The second of the activities covers techniques that are more specifically used for the aseptic processing of pharmaceuticals; this includes specific aseptic techniques such as how to assemble ingredients, spraying in and how to use vials, ampoules and blind hubs.
Multiple choice questions are interspersed throughout the package to test the user’s understanding of the material covered.
CONTENTS OF THE PACKAGE
Aseptic Processing consists of the following activities:
General:
- Sources Of Contamination
Control Of Contamination:
- Clean Rooms
- Clean Air Devices
- Personnel
- Environmental Monitoring
Aseptic Techniques
- General Microbiological Techniques
- Pharmaceutical Aseptic Processing
About The Activities
Sources Of Contamination
This activity looks at the range of sources of contamination, covering living contamination and both active and inactive non-living contamination. The activity discusses how personnel are the largest source of contamination and how some people may need to be excluded from Clean Rooms.
Clean Rooms
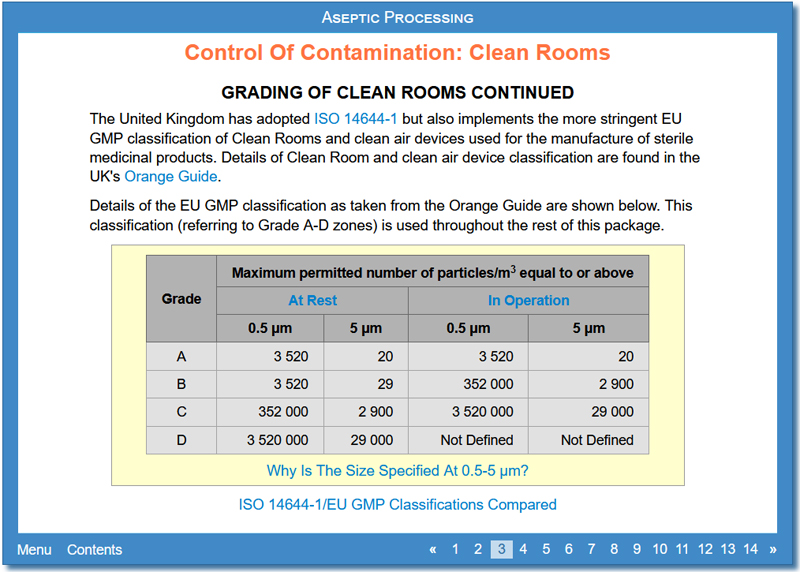
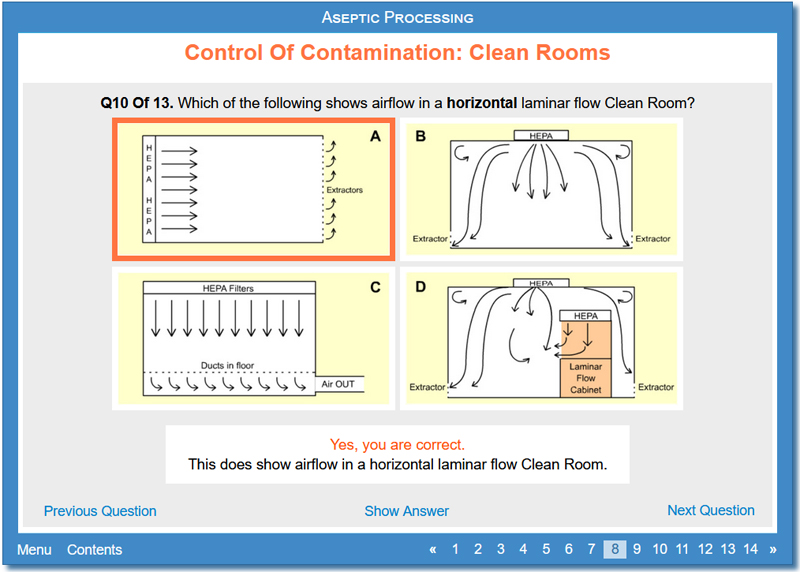
This activity discusses various aspects of Clean Rooms including what a Clean Room is, how Clean Rooms are classified (including ISO 14644-1), what is done in each grade of Clean Room for aseptic preparation, information about Clean Room design including materials used, shape and layout and air present, and how to minimise the introduction of contaminants.
Clean Air Devices
This activity discusses Horizontal Laminar Flow Cabinets and Vertical Laminar Flow Cabinets covering how they work, how they protect from contamination, how to work with them and how to clean them. The activity briefly covers isolators and biological safety cabinets.
Personnel
Personnel are the largest source of contamination. This activity looks at methods to control contamination arising from personnel including correct hand washing technique, use of protective clothing and adoption of good working techniques.
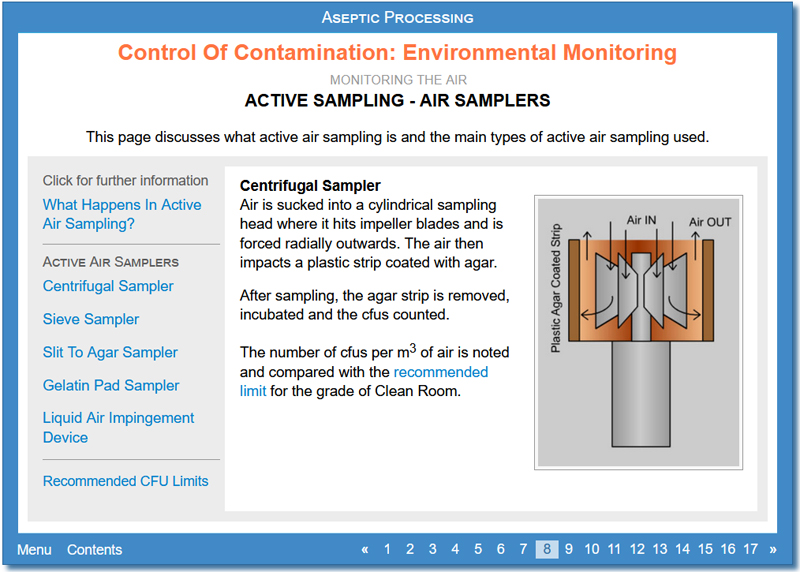
Environmental Monitoring
This activity looks at methods used to monitor the following environmental aspects of Clean Rooms:
- The air (for example checking overpressure, air flow and air change, and passive and active microbiological sampling techniques)
- The surfaces (for example contact pates, agar sausages and swabs)
- The personnel (for example fiver finger glove prints/finger dabs)
The activity finishes by briefly looking at broth fills.
General Microbiological Techniques
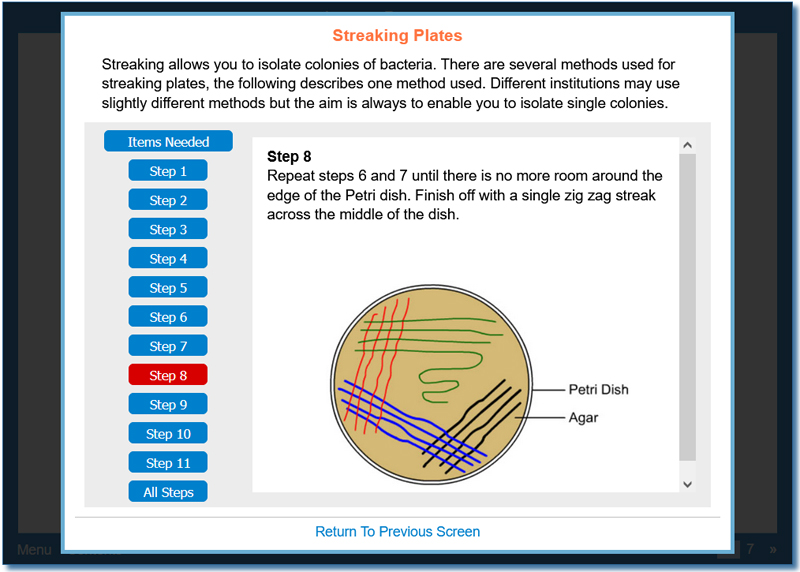
This activity introduces general aseptic handling techniques, used in several curricula including microbiology and pharmaceutical processing, that can be used by operators to minimise contamination. The first part of the activity looks at general practices that should be adopted when in aseptic preparation areas and when performing manipulations in aseptic preparation areas (for example the importance of labelling, how to minimise the production of aerosols). The activity continues by describing specific aseptic techniques – such as preparing and flaming an inoculation loop, removing a sample from a vessel, using pipettes, pouring plates and preparing bacterial lawns.
Pharmaceutical Aseptic Processing
The activity Pharmaceutical Aseptic Processing looks at aseptic techniques that are more specifically used for the aseptic processing of pharmaceuticals. The activity:
- Covers general information about the use of laminar flow cabinets
- Describes specific aseptic techniques including how to assemble ingredients, spraying in and how to use vials, ampoules and blind hubs
- Briefly covers the checking of products
- Briefly discusses some of the validation monitoring used for flow cabinets and pharmaceutical processing
The package covers information about contamination and aseptic processing and is suitable for users who need to learn about these aspects as part of a taught course and for users whose job may entail aseptic processing.
Pre-requisites: Users need to have no prior knowledge of aseptic processing although a knowledge of basic biology, basic laboratory apparatus (for example inoculation loops) and basic medical equipment (for example infusion bags) would be helpful.
Audience: Undergraduate students whose course includes aseptic work (possible courses include pharmacy, pharmacology, physiology, biology and microbiology); users whose job entails aspects of aseptic processing.
Time to complete: 2-4 hours
There are currently no reviews available for this package.
Share this page
Related resources
Main CoAcS website

The main CoAcS website explains more about the areas in which we operate including:
- pharmacy education
- healthcare applications to hospitals and pharma
- bespoke software
- pharmaceutical plant design and consultancy
College of Pharmacy (CoP)
The College of Pharmacy (CoP) website is the gateway to all of our CAL packages. Besides the subscribed pharmacy learning materials, you will also have free access to the:
- museum – containing images of historical documents and pharmacy artifacts
- library – with listings of the latest pharmacy journals and other publications
- lecture theatre – access to many diverse university presentations
- herb garden – to promote the understanding of herbs and medicinal plants and their place in traditional and modern pharmacy
Pharmaceutical Press agents
We are exclusive agents in the Middle East for the Pharmaceutical Press (the publishing arm of the Royal Pharmaceutical Society). We are able to supply countries with invaluable online resources – such as ‘Medicines Complete’. Many other titles are available including:
- the British National Formulary (BNF)
- Stockley’s Drug Interactions
- Martindale’s Complete Drug Reference
Please contact us directly if you wish to arrange a subscription.